Abstract
Risk factors for acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) in patients with multiple myeloma (MM) are not well understood. We have previously shown that monoclonal gammopathy of undetermined significance (MGUS) is associated with an excess risk of AML/MDS, indicating an inherent excess risk of AML/MDS in plasma cell disorders. In addition, randomized studies have shown that lenalidomide maintenance is associated with increased relative risk of AML/MDS. The excess risk of AML/MDS in lenalidomide treated patients has been found to be most prominent in patients that also receive alkylating agents. A recent randomized clinical study shows that patients undergoing delayed autologous transplant have the same overall survival as patients undergoing upfront autologous transplant with high dose melphalan at 3 years of follow-up. Also emerging data shows that patients with minimal residual disease (MRD) negativity have very similar progression free survival independent of therapy. Thus, patients treated with modern combination therapy who obtain MRD negativity might not have additional benefit from upfront autologous transplant. We were motivated to better define the relationship of alkylating therapy to risk of secondary malignancies.
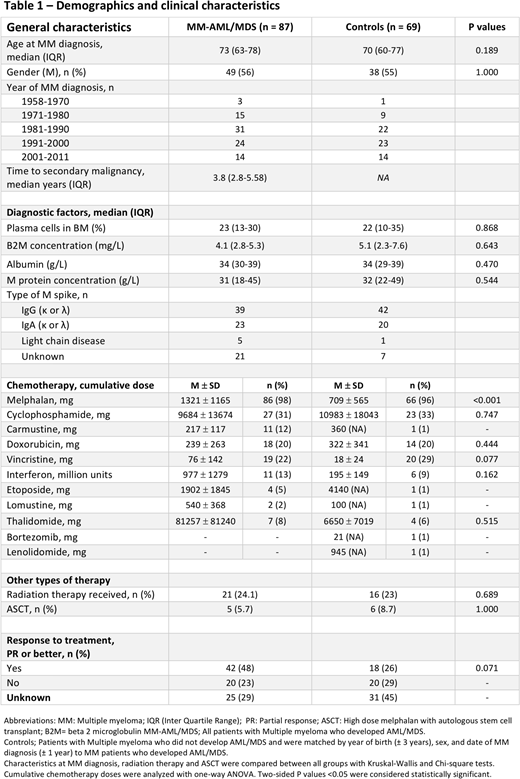
Information was obtained regarding all subsequent hematologic malignancies in all patients diagnosed with MM from January 1, 1958 to December 31, 2011 identified from the Swedish Cancer Registry. Each patient with MM and a subsequent AML/MDS diagnosis (i.e., cases) was matched to another MM patient without AML/MDS for year of birth, sex, and date of MM diagnosis (i.e., control group). Detailed information on baseline characteristics at diagnosis and treatment was collected from medical records. Characteristics at MM diagnosis, radiation therapy, and high dose melphalan with autologous stem cell transplant (ASCT) were compared between groups with Kruskal-Wallis and Chi-square tests. Cumulative chemotherapy doses were analyzed with one way ANOVA. Post hoc analysis with Fisher´s least significant difference (LSD) was performed.
A total of 26,627 patients were diagnosed with MM in Sweden during the study period. Of these, 113 patients developed subsequent AML/MDS. Data was found for 87 and they were matched to 69 controls. The demographic and clinical characteristics for both groups are listed in Table. The median age at MM diagnosis was 73 years (38 females and 49 males) for cases and 70 years (31 females and 38 males) for controls. The median time from MM diagnosis to AML/MDS diagnosis was 3.8 years (IQR 2.8 - 5.8). The mean cumulative melphalan dose was significantly higher (1,321 mg , SD ± 1,165 mg) for cases, than for the controls (709 mg , SD ± 565 mg)(p= <0.001). Post hoc analysis confirmed that cases had a statistically significant higher mean cumulative melphalan dose compared to controls, p <0.01.
In this large nationwide population based study including almost 27,000 MM patients diagnosed during over >50 years period in Sweden, we confirm our previous preliminary findings that higher mean cumulative dose of melphalan was associated with increased risk of developing AML/MDS. With the average myeloma patient living over 10-15 years from diagnosis, strategies to avoid secondary complications is becoming more important. Our results showing that melphalan is associated with an increased risk of AML/MDS, call for studies using response driven (i.e. MRD based) therapy in myeloma; and high dose melphalan is rather offered to patients who are MRD positive, and/or as relapse therapy.
Landgren:Pfizer: Consultancy; Karyopharm: Consultancy; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal